When a greenie says a project or process is “So-o energy-intensive,” we are expected to give up and buy a bicycle. Or a train ticket.
That’s not necessary.
Chemical engineers know some very cunning tricks for recycling heat and pressure in thermochemical processes. I saw some of their wizardry up close at a hydrothermal liquefaction (“HTL”) plant in Christchurch. The plant was manufacturing synthetic petroleum from wet biomass such as grass clippings and algae. They had proven they could make good quality petrol and diesel without using so much as a teaspoon of fossil petroleum.
The HTL process uses temperatures as high as 400 °C and pressures up to 340 atmospheres. I really (REALLY) wanted to know how you could heat up and pressurise the raw material and then cool down the finished product without burning up so much energy you’d go broke.
It turns out to be surprisingly easy, when you know how.
The process runs continuously, with a soup of biomass going in one end of the processing plant and synthetic petroleum coming out the other end. Imagine this happens inside a pipe: One-half of the pipe contains liquid that is gradually warming up on its way to the part where the chemical reaction takes place, and the other half of the pipe contains liquid which has been through the reaction chamber and is gradually cooling down.
The trick is to fold this pipe back on itself, and arrange it so that the liquid coming out warms up the liquid going in. This folded pipe is called a “counterflow heat exchanger”: Heat is exchanged between the liquid flowing outwards, and the liquid flowing inwards.
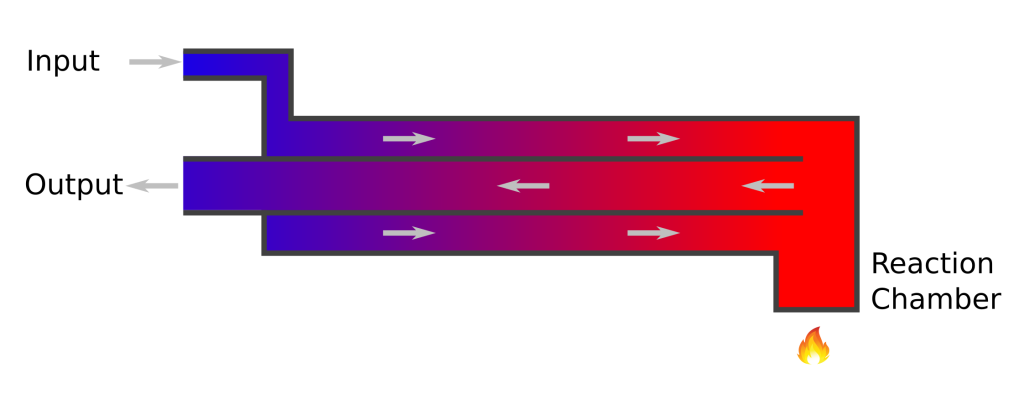
The HTL system I saw in Christchurch used a concentric design. Raw material flowed along the outer tube, and finished product flowed through the inner tube. The incoming material absorbs heat from the finished product, gradually warming up as it flows toward the reaction chamber. A well-designed heat exchanger of this type should recycle at least 95% of the heat from the finished product to the raw material. The reaction chamber needs only a tiny amount of heat to maintain the correct operating temperature.
Balancing the pressure is also very simple. The reactor I saw in Christchurch used a pair of pistons, each running in its own cylinder. Each cylinder has a pair of valves controlling the flow of raw material and finished product. The input piston pushes raw material through a valve into the input side of the heat exchanger. The output piston allows finished product to flow out into the output cylinder. The pistons balance like a see-saw. It takes very little energy to push raw material into the reactor, no matter how intense the operating pressure, because the cylinders are coupled together. When the input piston moves inward, the output piston moves outward.
As soon as I saw this balanced heat exchange system in operation, I realised how important it is to think about the complete system. On paper, a critic can make HTL look impossibly uneconomic, while an enthusiast can prove it’s a very good way to convert grass clippings into petrol. It all depends on how effectively the process recycles heat and pressure.
Experimental setups in laboratories seldom try to recycle heat or pressure. That’s not surprising because early-stage laboratory studies may be designed purely to test a process’s basic chemistry. If the system looks promising, engineers will build a “pilot” or “demonstration” plant that will give a better idea of the potential economics. That’s when heat and pressure recycling become important. Expect to find balanced pressure swing systems and counterflow heat exchangers in pilot or demonstration plants. Don’t expect to find them in early-stage lab experiments.
Heat and pressure recycling systems, also known as thermodynamic swing processes, are quite common in renewable fuel production, and in systems that harvest carbon dioxide from air or water. Renewable fuel production and carbon dioxide harvesting are vital to climate engineering technologies.
The next time you read about a process or technology that: “Can’t possibly be commercialised because it is far too energy intensive”, take a deep dive into the details. Have the critics analysed a well-designed system that recycles process heat and pressure? If not, if the critics have looked only at a system that does not recycle heat and pressure, the criticism is not valid. In that case, keep an eye out for further developments. If it’s a promising system, it’s quite likely that someone will come up with an economic design.
Another option is to get stuck in and develop the system yourself. You might just hit on something vitally important.
We are technorg.
